what is required for ketamine to induce psychotic symptoms?
Introduction
The use of constructed drugs such as methamphetamine and ketamine has been recognized as an increasingly serious and of import public health consequence in China and other countries (ane–three). A serial of studies signal that chronic use of both methamphetamine and ketamine tin atomic number 82 to psychotic symptoms (iv–6), which are mainly manifested as persistent hallucinations, delusions, and negative symptoms.
The experience of psychotic symptoms ranged from 26 to 46% among people with methamphetamine use disorder (MUD) (7). A study with 292 MUD patients from a drug rehabilitation center in Malaysia showed that 47.9% of them had a history of psychotic symptoms (8). Studies show that patients with ketamine use disorder (KUD) have a relatively lower risk of psychotic symptoms than those of MUD patients, effectually 8% (ix). In drug-costless salubrious individuals, low-dose administration of either ketamine or amphetamine produced positive symptoms (such as conceptual disorganization) and euphoria. Ketamine mainly produced perceptual changes, concrete ideation, and unusual mannerisms, negative symptoms, and disrupted delayed recall. Amphetamine mainly produced hostility, grandiosity, and somatic business. The findings from the above study indicate that glutamate and DA may differentially contribute to psychosis (x). A contempo systematic review and meta-analysis further confirmed that ketamine induced psychosis-like symptoms in healthy volunteers (11).
The neuropathological mechanisms of psychotic symptoms are not fully understood. Both methamphetamine and ketamine utilise-induced psychotic symptoms tin mimic symptoms of schizophrenia. Methamphetamine has been used for the study of the dopamine (DA) model of schizophrenia (12). Ketamine, every bit an N-methyl-D-aspartate (NMDA) antagonist, has ofttimes been studied for the glutamate model of schizophrenia (13). DA plays a well-recognized role in a multifariousness of neurophysiologic functions such as knowledge, mood and reward (fourteen). Methamphetamine dysregulates both DA manual and DA reuptake, and it mainly acts on DA transporter (DAT) and the vesicular monoamine transporter 2 (VMAT2) to inhibit the reabsorption of DA and promote the release of DA in the Nucleus Accumbens (NA) via the sigma receptor (xv). Ketamine primarily impairs NMDA glutamate receptor neurotransmission, and it has been suggested to increase cortical glutamate release via indirect inhibition of GABAergic interneurons, implicating in the pathophysiology of schizophrenia (16).
Although a line of study has shown that the psychotic symptoms acquired by methamphetamine and ketamine are similar to those of schizophrenia. The prevalence and detailed features of psychotic symptoms (including positive, negative and general symptoms) among methamphetamine utilize disorder (MUD) and ketamine use disorder (KUD) patients are largely unknown. The aim of this study was to measure psychotic symptoms among patients with MUD and KUD. Based on previous studies, this study causeless that the prevalence of positive and negative symptoms would be higher among MUD patients than KUD patients. The findings from this study may provide a preliminary footing for clinical interventions of MUD and KUD associated psychosis, and requite a new perspective for exploring the neuropathological mechanism of psychotic symptoms of schizophrenia.
Methods
Recruitment
This cross-sectional study assessed the prevalence of psychotic symptoms in a clinic-based sample of KUD patients and MUD patients. We recruited 380 KUD patients and 462 MUD patients from ii rehabilitation centers in china (Guangzhou Baiyun Voluntary Drug Rehabilitation Center in Guangdong Province and Kangda Voluntary Drug Rehabilitation Middle in Hunan Province) from January 2012 to October 2016. Drug use (methamphetamine or ketamine) was validated via urine toxicology screen and self-report data. All participants were diagnosed through a semi-structured interview (Structured Clinical Interview for DSM-IV-TR, Centrality I, Patient Version, SCID-I/P) by experienced psychiatrists (with more than 5 years of clinical experience). The Positive and Negative Syndrome Scale (PANSS) was used to appraise psychotic symptoms in these 2 groups of patients. Earlier enrolling patients into this written report, all psychiatrists who involved in electric current study were trained to administer the PANSS and SCID-I/P with skillful reliability (>0.80). The inclusion criteria were: inpatients from drug rehabilitation centre; afterwards detoxification and had two weeks of drug abstinence; met diagnosis for MUD or KUD; used methamphetamine or ketamine for more 12 months; eighteen years of age or older; willing to participant, being able to communicate in Chinese. The exclusion criteria were: suffering from other severe mental and/or concrete illnesses; have a history of brain trauma; and have other substance use disorders (except for nicotine).
Ethics
Informed consent was obtained from all patients in the written report. The procedures were performed in accord with the Announcement of Helsinki. The upstanding blessing for this study was obtained from the Ethics Committee of the Second Xiangya Infirmary, Central South University (No. S163, 2011).
Measures
Sociodemographic and Drug Use Data
Sociodemographic and drug use information, such as age, gender, education, historic period of the first use, quantity of drug (g) per time, drug craving, and times of rehabilitation were collected past self-reported questionnaires. Methamphetamine or ketamine craving was assessed by the Visual Analog Calibration for Craving (VASc) (17). The VASc displays a scale from 0 (left, no peckish) to 10 (right, about extreme craving).
The Positive and Negative Syndrome Calibration
The Positive and Negative Syndrome Calibration (18) is a 30-detail scale designed to obtain a measure of positive (items P1 to P7) and negative (items N1 to N7) symptoms in schizophrenic patients, likewise as a mensurate of full general psychopathology (items G1 to G16). All 30 items are rated on a 7-point scale: 1 = absent, 2 = minimal, 3 = mild, 4 = moderate, 5 = moderate–severe, 6 = severe, vii = extreme. Factorial analyses of subscales institute that a five-gene model better captures PANSS construction in schizophrenia samples (19). In the five-gene model, smaller groupings of items represent the following symptoms: positive (items P1, P3, P5, G9), negative (items N1, N2, N3, N4, N6, G7), disorganized/concrete (items P2, N5, G11), excited (items P4, P7, G8, G14), and depressed (items G2, G3, G6) symptoms.
Statistical Analyses
Statistical analysis was performed using the SPSS for Windows (Version 23, SPSS Inc., Chicago, IL, U.s.a.) software bundle. Descriptive statistics were used to examine demographic, drug utilise, and psychotic characteristics. Contained sample t-tests or χ2-square tests were performed to determine group differences in demographic characteristics, substance use profiles, and psychotic symptoms between these two groups. Multiple linear regression models have been applied to evaluate the impact of types of used drugs (methamphetamine or ketamine), gender, age, age of commencement-fourth dimension drug apply, duration of drug employ (months), and frequency during the concluding drug use month on psychotic symptoms (PANSS total score). An blastoff level of 0.01 was gear up to determine statistical significance.
Results
Sociodemographic and Drug Use Characteristics
This study included 842 drug users (462 MUD patients and 380 KUD patients). Demographic and drug use characteristics of patients with MUD and KUD are shown in Table 1.
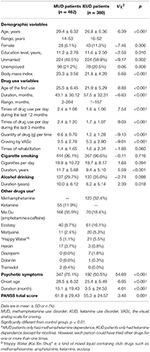
Table 1. Sample characteristics.
Factors Predicting Psychotic Symptoms
Multiple linear regression models were applied to evaluate the predictors (demographic and drug utilise factors) for psychotic symptoms (PANSS total score). As shown in Tabular array ii, but types of used drugs (methamphetamine or ketamine) were associated with PANSS full score, indicating that MUD patients experienced more psychotic symptoms than KUD patients did (p < 0.001).
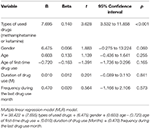
Table two. MLR model predicting increment in PANSS full score.
Psychotic Symptoms in MUD Patients and KUD Patients
A full of 75.1% (due north = 347) MUD patients and l.v% (n = 192) KUD patients experienced psychotic symptoms (Tabular array 1). Compared with KUD patients, MUD patients were more likely to endure from psychotic symptoms (95% CI: 3.532–11.858, p < 0.001). The scores of PANSS were compared between these two groups. We notice that compared with KUD patients, MUD patients were more probable to experience positive symptoms (xi.v ± 6.07 vs. 15.1 ± 8.22, P < 0.001) and negative symptoms (12.4 ± six.60 vs. 14.v ± viii.63, P < 0.001), but not general symptoms (31.2 ± xiii.90 vs. 32.2 ± 15.13, P = 0.331) (Table 3). The proportion of each symptom in both groups, including each item of the PANSS positive, negative, and general symptoms, are likewise shown in Table iii. A five-gene model of positive, negative, disorganized/concrete, excited, and depressed symptoms was also compared between 2 groups (Table 4).
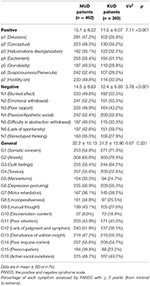
Table 3. Positive, negative, and general symptoms assessed by PANSS between 2 groups.

Table 4. PANSS scores past the 5-factor model between 2 groups.
Give-and-take
Principal Findings
The current report assessed the prevalence and detailed features of psychotic symptoms among MUD and KUD patients. This study establish that 75.1% MUD patients and 50.v% KUD patients from drug rehabilitation centers in China experienced psychotic symptoms, indicating that MUD patients are more than likely to endure from psychotic symptoms (including positive and negative symptoms) than KUD patients. However, MUD patients and KUD patients showed no group differences in overall full general symptoms.
Psychotic Symptoms in MUD Patients and KUD Patients
Compared with previous studies, our report found relatively high prevalence of psychotic symptoms in MUD patients and KUD patients, especially in MUD patients. One report reported that the prevalence of psychotic symptoms ranged from 26 to 46% in MUD patients (seven). Another study reported that 47.ix% MUD patients had history of psychotic symptoms (8). A written report constitute that only 8% KUD had psychotic symptoms (9). However, our written report found more than half of KUD patients and ii-thirds of MUD patients experienced psychotic symptoms.
This study found that, compared with KUD patients, MUA patients were more probable to experience both positive and negative symptoms, but not general symptoms. Previous studies suggest that methamphetamine mainly causes psychotic symptoms by causing DA system dysfunction, acting on DA transmission in the central nervous organisation via the inhibition of the DA transporter and the VMAT2, and leading to the increase of DA concentration in the mesolimbic, nigrostriatum, and mesocortical, resulting in psychotic symptoms, mainly positive symptoms (half-dozen, 20). Furthermore, in the nigrostriatum, dysregulated DA neuron firing can abnormally highlight irrelevant stimuli, thereby producing percepts and thoughts with aberrant salience, resulting to delusions and hallucinations (10, 21). In terms of methamphetamine use induced negative symptoms, 1 possible explanation is that the reduction of the bespeak-to-noise ratio of adaptive phasic signaling has the potentiality to reduce the appetitive backdrop of a given reward, thereby leading to motivation and anhedonia, and other negative symptoms.
Research indicates that ketamine produces psychotic symptoms through dysfunction in the glutamate system. As a high affinity non-competitive antagonist of NMDAR, ketamine has been shown to mimic more negative symptoms than active symptoms of psychotic behavior in previous studies (ten, 21). A line of study found that ketamine can produce positive and negative symptoms like to schizophrenia with long-term employ (22, 23), which is consistent with our results. A preclinical study found that ketamine causes psychotic episodes by significantly increasing synaptic glutamate release in the cortex and striatum (24).
Loftier prevalence of psychosis amid MUD patients and KUD patients in current study indicates that both the glutamate and DA systems directly or indirectly interact with psychotic symptoms (4, 10, 13). The psychotic symptoms were more easily induced past MA than ketamine, indicating that the dysfunction of the glutamate system may cause psychotic symptoms through indirect (not direct) action on the DA system. Methamphetamine or amphetamine induced hyper-dopaminergic states would be associated with the more vulnerability of psychosis (25). Previous studies proved that modulation of the DA arrangement affects cortical glutamate levels (mainly in the prefrontal lobe) and local glutamate release; glutamate levels tin affect DA release in the striatum; and the administration of ketamine can pb to the increase of DA release in the striatum. Low glutamate release or insufficient activation of NMDA receptors on cortical GABA interneurons may lead to hyperactive striatal dopaminergic activity (xiii, 26–28). Clinically, the use of DA receptor antagonists for ketamine-induced psychosis further provides bear witness of the interaction of glutamate and DA (29). Thus, nosotros speculate that the glutamate system tin can use a mutual pathway that interacts with the DA system to contribute to the occurrence of psychotic symptoms.
In short, comparing psychotic symptoms induced by the nonmedical use of ketamine and methamphetamine, nosotros have demonstrated that some features of psychotic symptoms in MUD and KUD patients are highly similar to schizophrenia. Our study indicates the significance of identifying and treating psychotic symptoms in MUD and KUD patients, and provides a preliminary basis for clinical identification of the feature of psychotic symptoms between schizophrenia and substance utilize disorder.
Limitations
This study has some limitations that need to exist considered. First of all, we only assessed residual psychotic symptoms just not astute effects of methamphetamine and ketamine. All participants were from voluntary drug rehabilitation centers, and they were non randomly sampled, so the representativeness is limited. Secondly, most patients in the present study were male, then we did not appraise gender differences for psychotic symptoms. Thirdly, some drug utilize variables were different between MUD patients and KUD patients. Compared with MUD patients, KUD patients showed an earlier age of the first employ, longer elapsing of ketamine use, higher quantity of drug use per time, and higher drug craving level. Nevertheless, this study establish more MUD patients experienced psychotic symptoms than KUD patients. The underlying mechanism needs to be farther researched. Lastly, some MUD or KUD patients also used other drugs. Nonetheless, this study excluded patients with other substance utilise disorders (excluding nicotine).
Decision
In decision, this written report found that psychotic symptoms are commonly reported past MUD patients and KUD patients from drug rehabilitation centers, and that MUD patients are more likely to suffer from psychotic symptoms (but not general symptoms) than KUD patients. These findings betoken the importance of assessing psychotic symptoms amongst these ii groups of patients. It also provides a new perspective for exploring glutamatergic model and dopaminergic model of psychosis.
Data Availability Argument
The raw data supporting the conclusions of this commodity will be fabricated available by the authors, without undue reservation.
Ethics Statement
The study protocol has been approved by Ethics Commission of the Second Xiangya Infirmary, Central Due south University (No. S163, 2011). The patients/participants provided their written informed consent to participate in this report.
Writer Contributions
YL conceived the study and took the atomic number 82 in writing the manuscript. YL and MX did the literature review. TL did the statistical analyses. YL, TL, and MX drafted the report. YL, CQ, and QW collected the data. TL, MX, and JT interpreted the data and commented on the manuscript. JT supervised the written report. All authors contributed to the article and approved the submitted version.
Funding
This report was supported by the National Natural Science Foundation of China (Grant No. 81671325 to YL) and the Hunan Provincial Natural Scientific discipline Foundation of People's republic of china (Grant No. 2020JJ4794 to YL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could exist construed as a potential disharmonize of interest.
Publisher'southward Note
All claims expressed in this commodity are solely those of the authors and do not necessarily stand for those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may exist evaluated in this article, or merits that may be made past its manufacturer, is not guaranteed or endorsed past the publisher.
Acknowledgments
We acknowledge all the professionals from Voluntary Drug Rehabilitation Centers who helped a lot in data collection. The authors thank all the patients who participated in this study.
References
1. Du P, Li K, Li J, Xu Z, Fu 10, Yang J, et al. Methamphetamine and ketamine apply in major Chinese cities, a nationwide reconnaissance through sewage-based epidemiology. Water Res. (2015) 84:76–84. doi: 10.1016/j.watres.2015.07.025
PubMed Abstruse | CrossRef Total Text | Google Scholar
iv. Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cerebral impairment in neuroleptic-costless schizophrenics. Neuropsychopharmacology. (1997) 17:141. doi: 10.1016/S0893-133X(97)00036-5
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi Chiliad, Parent D, et al. Psychiatric symptoms in methamphetamine users. Am J Aficionado. (2004) 13:181–90. doi: 10.1080/10550490490436055
PubMed Abstract | CrossRef Total Text | Google Scholar
6. Bramness JG, Gundersen OH, Guterstam J, Rognli EB, Konstenius M, Loberg EM, et al. Amphetamine-induced psychosis–a separate diagnostic entity or chief psychosis triggered in the vulnerable? BMC Psychiatry. (2012) 12:221. doi: x.1186/1471-244X-12-221
PubMed Abstract | CrossRef Full Text
8. Sulaiman AH, Said MA, Habil MH, Rashid R, Siddiq A, Guan NC, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. (2014) 55 Suppl 1:S89–94. doi: 10.1016/j.comppsych.2013.01.003
PubMed Abstract | CrossRef Full Text | Google Scholar
nine. Fan N, Xu Thousand, Ning Y, Rosenheck R, Wang D, Ke X, et al. Profiling the psychotic, depressive and anxiety symptoms in chronic ketamine users. Psychiatry Res. (2016) 237:311–five. doi: 10.1016/j.psychres.2016.01.023
PubMed Abstract | CrossRef Total Text | Google Scholar
10. Krystal JH, Perry EB Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive role. Arch Gen Psychiatry. (2005) 62:985–95. doi: ten.1001/archpsyc.62.9.985
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Beck K, Hindley G, Borgan F, Ginestet C, McCutcheon R, Brugger S, et al. Association of ketamine with psychiatric symptoms and implications for its therapeutic use and for understanding schizophrenia: a systematic review and meta-assay. JAMA Netw Open. (2020) 3:e204693. doi: 10.1001/jamanetworkopen.2020.4693
PubMed Abstruse | CrossRef Full Text | Google Scholar
12. Grant KM, LeVan TD, Wells SM, Li M, Stoltenberg SF, Gendelman HE, et al. Methamphetamine-associated psychosis. J Neuroimmune Pharmacol. (2012) 7:113–39. doi: 10.1007/s11481-011-9288-ane
PubMed Abstract | CrossRef Full Text | Google Scholar
fourteen. German CL, Baladi MG, McFadden LM, Hanson GR, Fleckenstein AE. Regulation of the dopamine and vesicular monoamine transporters: pharmacological targets and implications for disease. Pharmacol Rev. (2015) 67:1005–24. doi: ten.1124/pr.114.010397
PubMed Abstruse | CrossRef Total Text | Google Scholar
15. Hedges DM, Obray JD, Yorgason JT, Jang EY, Weerasekara VK, Uys JD, et al. Methamphetamine Induces Dopamine Release in the Nucleus Accumbens Through a Sigma Receptor-Mediated Pathway. Neuropsychopharmacology. (2018) 43:1405–fourteen. doi: 10.1038/npp.2017.291
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Stone JM, Dietrich C, Edden R, Mehta MA, de Simoni S, Reed LJ, et al. Ketamine furnishings on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. (2012) 17:664–5. doi: 10.1038/mp.2011.171
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus v-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. (2012) 137:246–50. doi: ten.1016/j.schres.2012.01.031
PubMed Abstract | CrossRef Total Text | Google Scholar
21. Stone JM, Erlandsson K, Arstad E, Squassante L, Teneggi V, Bressan RA, et al. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [(123)I]CNS-1261 SPET written report. Psychopharmacology. (2008) 197:401–eight. doi: ten.1007/s00213-007-1047-x
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Moghaddam B, Krystal JH. Capturing the affections in "affections grit": 20 years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. (2012) 38:942–ix. doi: 10.1093/schbul/sbs075
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Lisek Thousand, Ferenc B, Studzian M, Pulaski 50, Guo F, Zylinska 50, et al. Glutamate Deregulation in Ketamine-Induced Psychosis-A Potential Role of PSD95, NMDA Receptor and PMCA Interaction. Front Cell Neurosci. (2017) 11:181. doi: x.3389/fncel.2017.00181
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Cassidy CM, Balsam PD, Weinstein JJ, Rosengard RJ, Slifstein Yard, Daw ND, et al. A perceptual inference mechanism for hallucinations linked to striatal dopamine. Curr Biol. (2018) 28:503–14.e4. doi: x.1016/j.cub.2017.12.059
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation past D1 receptors in prefrontal circuits. Proc Natl Acad Sci U South A. (2001) 98:295–300. doi: 10.1073/pnas.98.one.295
PubMed Abstract | CrossRef Total Text | Google Scholar
27. Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. (2014) 167:98–107. doi: 10.1016/j.schres.2014.12.026
PubMed Abstract | CrossRef Total Text | Google Scholar
29. Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. (2020) 172:107704. doi: x.1016/j.neuropharm.2019.107704
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.786622/full
0 Response to "what is required for ketamine to induce psychotic symptoms?"
Post a Comment